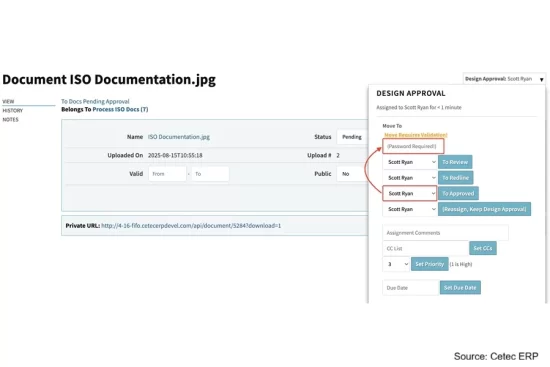
Cetec ERP released versions 4.19 and 4.20 designed with new capabilities to support the day-to-day compliance and quality needs of medical device and life science companies.
Related Posts
Investing in Skills Today Ensures a Competitive Edge Tomorrow
The transition from high school to the workforce is vital for young adults. While many pursue four-year degrees,…
Understanding Reverse Engineering in Quality Control and Inspection
Reverse engineering helps engineers understand product design and functionality by dissecting products, aiding in creating or improving similar…
The Next Frontier of Automation: Quality Assurance in an AI-Driven Era
By 2025, automation technologies such as AI-driven inspection systems and autonomous robots are essential for manufacturers, enhancing productivity…




